Manufacture of Soluble Silicates
Production routes
Sodium and potassium silicate glasses (lumps) are produced by the direct fusion of precisely measured portions of pure silica sand (SiO2) and soda ash (Na2CO3) or potash (K2CO3) in oil, gas or electrically fired furnaces at temperatures above 1000°C according to the following reaction:
M2CO3 + x SiO2 -> M2O . x SiO2 + CO2 (M = Na, K)
The Furnace Route
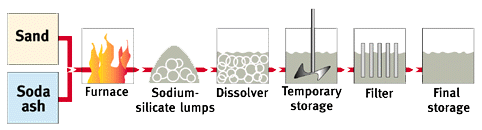
Solutions of soluble silicates ("waterglass") may be produced either by dissolving the soluble silicate lumps in water at elevated temperatures (and partly at elevated pressure) or for certain qualities also by hydrothermally dissolving a reactive silica source (mainly silica sand) in the respective alkali hydroxide solution according to the equation:
2 MOH + x SiO2 -> M2O . x SiO2 + H2O
The Hydrothermal Route
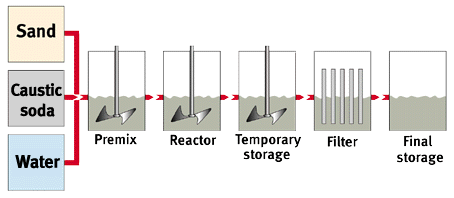
In general, solutions are subsequently filtered to remove any residual turbidity and adjusted to yield products to a particular specification.
Amorphous silicate powders are made by drying aqueous solutions by means of spray or drum dryers. These products may then be further treated in order to modify powder properties, e.g. particle size, bulk density.
Crystalline alkali silicate powders of a specific composition but containing different amounts of water of crystallisation can be produced by various routes; e.g. sodium metasilicate pentahydrate
(Na2SiO3 . 5H2O) is normally produced by blending sodium silicate solutions and additional caustic soda (NaOH) to achieve a mother liquor with WR SiO2 : Na2O = 1.0, from which the final product is crystallised. Anhydrous metasilicate can be prepared via the furnace route.
The products are then separated, sieved and processed as required.
Raw materials
The main raw materials for the production of soluble silicates are quartz sand (or other silica sources), alkali carbonates (e.g. soda ash Na2CO3, potash K2CO3), alkali hydroxides (e.g. NaOH, KOH, LiOH), water and fuels / energy, (e.g. oil, gas, electricity). Filter aids (mostly from natural sources) may also be used.


